Abstract
Introduction Blood product transfusion continues to retain a critical role in the supportive care of patients with acute myeloid leukemia (AML) particularly during the initial phase of intensive induction chemotherapy. Whereas previous studies have shown increased transfusion dependency to portend inferior outcome, clinical and disease features predictive of an increased transfusion burden and the overall prognostic impact of transfusion support in this setting have not been determined as of yet. Herein, we evaluated the various blood product components administered during induction aiming to delineate current transfusion practices in AML patients.
Methods We reviewed the medical records of 180 consecutive adult patients with newly diagnosed AML, who were treated with intensive chemotherapy from December 2014 through January 2022 at the Sheba Medical Center. Clinical data extraction and exploration were done with the MDClone big data platform. All patients assessed in the study received standard anthracycline and cytarabine-based intensive induction chemotherapy or CPX-351. Patients received irradiated and leukodepleted blood products. Alloimmunization was defined according to the National Heart, Lung, and Blood Institute working group criteria.
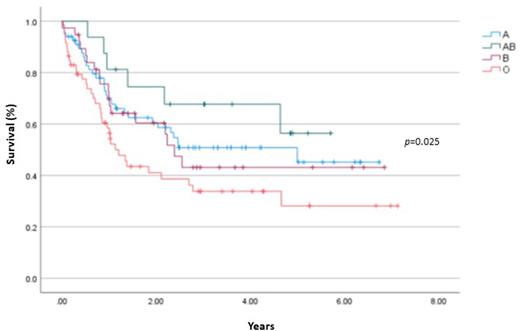
Results The analysis comprised 180 patients with a median age of 57 years (range 19-77) with 80% designated as de novo AML and the remainder as secondary AML. Fifty-four patients (31%) were FLT3-ITD mutated and 73 patients (42%) harbored NPM1. Favorable risk and intermediate risk ELN 2017 patients accounted for 43% and 34% of patients, respectively. The median number of red blood cell (RBC) and platelet units given during induction were 9 units and 7, respectively. Seventeen patients (9%) received cryoprecipitate and fresh frozen plasma (FFP) was given to 12 patients (7%). Lower initial hemoglobin and platelet levels were predictive of increased use of RBC (p<0.0001) and platelet transfusions (p<0.0001). Use of FFP was significantly associated with induction related mortality (42% versus 5%; p<0.0001) and with FLT3-ITD status (72% versus 28%; p=0.004). Transfusion associated alloimmunization was seen in 7 patients (4%) with patients with lower mean initial platelet counts being more likely to experience alloimmunization (38 X 109/l versus 83 X 109/l; p=0.01). Alloimmunized patients had significantly higher RBC transfusion (14 units versus 9.5 units; p=0.012) and prestorage leukodepleted filtered RBC (6.1 units versus 1.6 units; p<0.0001) requirements during induction compared with non-alloimmunized patients. Blood group AB experienced improved mean overall survival compared to blood group O patients (4.1 years versus 2.8 years; p=0.025). In multivariate analysis, increased number of FFP [hazard ratio (HR), 4.23; 95% confidence interval (CI), 2.1-8.6; p<0.001) and RBC units (HR, 1.8; 95% CI, 1.2-2.8; p=0.008) given was associated with inferior survival.
Conclusion In this analysis we comprehensively mapped the transfusion trajectory of AML patients during induction therapy and identified clinically meaningful associations impacting the need for the various blood components as well as overall survival. Transfusion needs during induction crucially impact the clinical trajectory of AML patients.
Disclosures
Avigdor:Takeda, Gilead, Novartis, Roche, BMS: Consultancy; AbbVie: Honoraria. Canaani:AbbVie: Consultancy; Astellas: Consultancy; Medison: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal